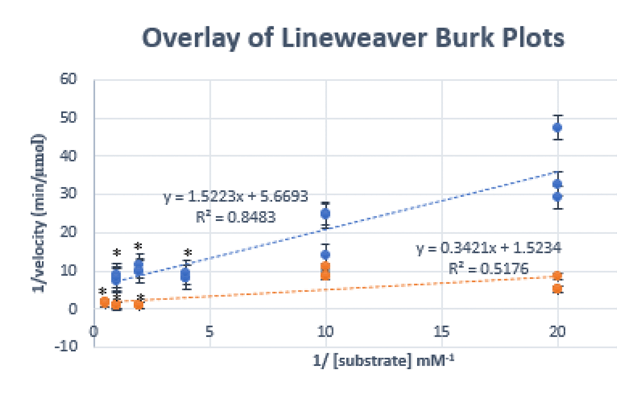
Human placental alkaline phosphatase (AP) is an enzyme that catalyzes the removal of phosphate and it can be inhibited by L-Phenylalanine (Hoylaerts et al., 1992). The purpose of the project is to investigate the kinetic parameters of human placental AP in the presence and absence of L-Phenylalanine. NPP was used as a substrate in order to get a colored product which allows the measurement of its concentration using spectrophotometry at 450 nm. We hypothesize that L-phenylalanine will be an efficient uncompetitive inhibitor of human placental AP, and that will be determined by a decrease in Vmax and Km upon inhibition, and calculated from the Lineweaver-Burke Plot. The results show that the Km value decreased in the presence of 50mM L-Phenylalanine as expected for uncompetitive inhibition. Km insignificantly decreased from 0.269 ± 0.051 mM to 0.224 ± 0.141. However, the Vmax showed a significant increase in the presence of the inhibitor from 0.176 ± 0.180 (µmol/min) to 0.656 ± 0.109 (µmol/min). These observations do not allow us to confirm that L-Phenylalanine is an uncompetitive inhibitor at the concentration of 50mM.